 |
|
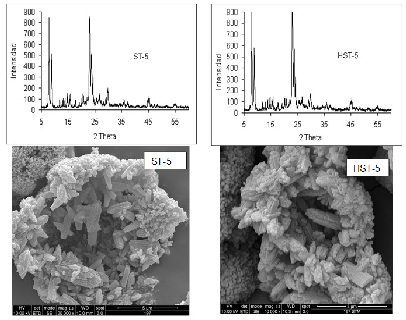
A synthesized and protonated MFI zeolite was modified by thermal and hydrothermal treatment and exchanged with nickel. The catalysts were characterized by different techniques and evaluated for the ethylene transformation at atmospheric pressure, 400 °C and space velocity equal to 7 h-1. The products were analyzed by gas chromatography, determining the conversion of ethylene (Xethylene) and product selectivity, consisting of paraffins, isomers, olefins, naphthenes and aromatics (PIONA). All zeolites without metal, showed good catalytic stability. The thermal and hydrothermal treatments carried out modify the textural properties of the solids. Nickel incorporation modifies ethylene conversion and catalyst stability. The effect of nickel on the PIONA selectivity depends on the treatment performed on the zeolite used as support. The combination of the treatments and the incorporation of nickel increase the selectivity towards C3 and C4 olefins.
Keywords: Ethylene transformation; hydrothermal treatment; MFI zeolite; thermal treatment; Ni-MFI.
|
|
 |

