 |
|
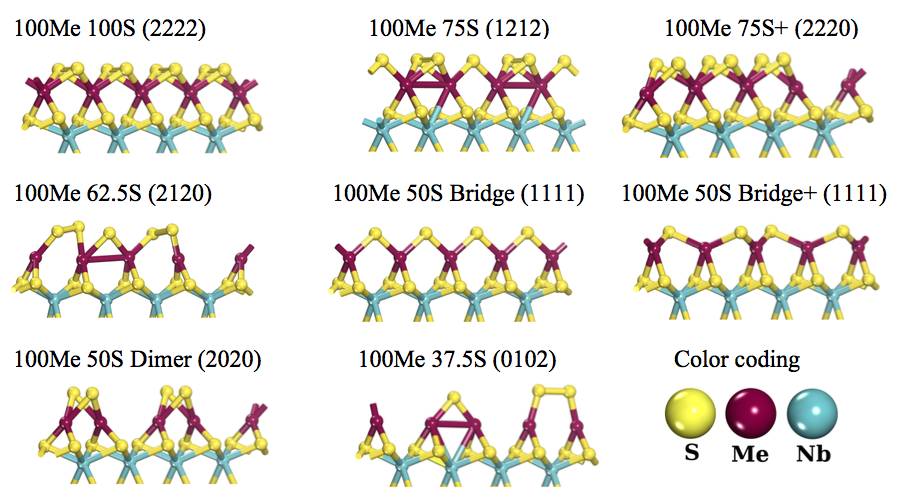
Density functional theory calculations of single-layer models of niobium sulfide catalysts promoted at the S-border by the transition metal atoms V, Cr, Fe, Co, Ni, and also by Cu are performed. Different degrees of promotion as well as of sulfidation are studied. The canonical potential for the sulfidation reaction for these models is obtained and the thermodynamic stability of these catalysts in terms of composition, temperature and pressure (pH2S/pH2) is examined. At working conditions (T=650K and pH2S/pH2=0.05) the most stable catalysts correspond to 100V75S and 100Cr100S which are structures where the promoter atoms fully replace the Nb atoms at the upper row of the single-layer crystal at the S-border. The potential activity of the active sites in these catalysts was studied by means of the surface electrostatic potential, Vs (r). The implications for the HDS reaction in hydrotreating conditions of these novel catalyst models is discussed.
Keywords: DFT, Canonical Potential, Promotor effect, Niobium Sulfide Catalysts, HDS.
|
|
 |

