 |
|
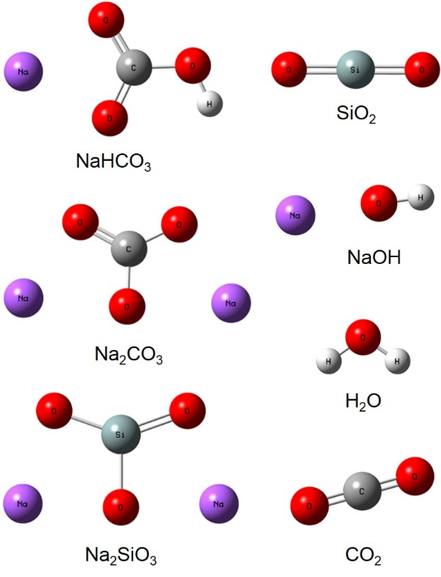
Sodium silicate has a wide range of applications from adhesives, lower carbon cements, cleaning compounds, deflocculants, protective coatings, soaps and detergents, silica-type catalysts and gels, pigments, etc. It is mainly obtained as a liquid, but a small fraction can be found in solid state. In this work, three different synthesis methods to produce crystalline sodium metasilicate are analized; the importance of synthesizing it in powder form is to be able to incorporate it into the productive processes of the cement industry, whose equipment is made for this purpose. Silicon dioxide in stoichiometric amounts was combined with each one of the following reactant: NaHCO3, Na2CO3 and NaOH. We present the most effective synthesis route to obtain sodium metasilicate based on a computational analysis and the results are validated experimentally. The characterization was carried out by X-ray diffraction and the Rietveld refinement, as well as by calorimetry and infrared and Raman spectroscopies. The experimental results were supported by molecular simulation, being both of them consistent. Using Na2CO3 as a reactant was the most effective reaction.
Keywords: sodium metasilicate; FTIR, Raman spectroscopy, thermal analysis; molecular simulation.
|
|
 |

