 |
|
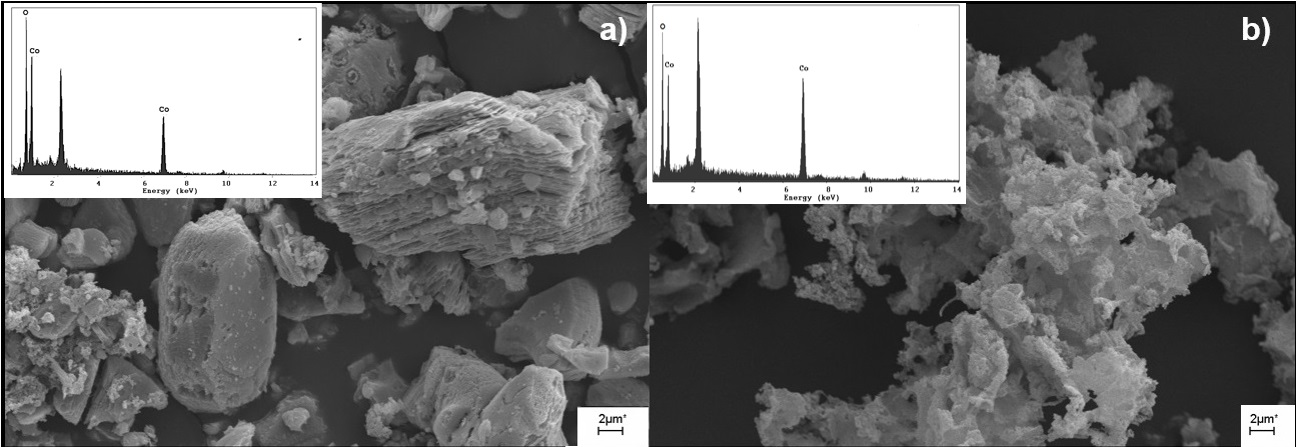
This work are shows the results of a comparative hydrometallurgical study of LiCoO2 leaching, obtained of the cathodes from spent lithium-ion batteries (LIBs), with HF and HF/H2O2. The operational parameters researched were H2O2 and HF concentrations, solid-liquid ratio, stirring speed, temperature and reaction time. The experimental results indicated that the best working conditions, with dissolution values close to 100%, were 348 K, 330 rpm, 30 min, 8 g/L, HF 0.6 M and H2O2 0.7 M. Thermodynamic study of the dissociation of HF in aqueous solution and EH-pH diagrams for Li-H2O, Co-H2O and Li-Co-F-H2O systems were performed. The results showed the feasibility of extracting and recovering metals contained in the sample.
Keywords: LiCoO2, LIBs, reducing agent, thermodynamic.
|
|
 |

