Revista Mexicana de Ingeniería Química, Vol. 18, No. 3 (2019), Bio327
Time-efficient pH-based method to quantify the activity of alkaline and halo-alkaline proteases
|
R.B. Martínez-Pérez, M.A. Camacho-Ruiz, I. Estrada-Alvarado, V. Armenta-Pérez, R.M. Camacho-Ruíz
https://doi.org/10.24275/rmiq/Bio327
Abstract
 |
|
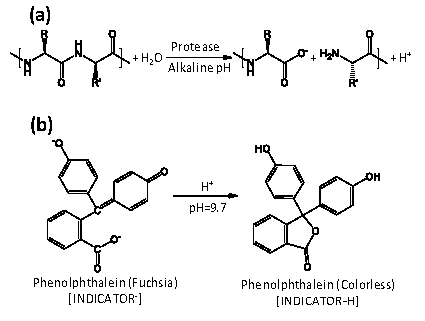
Proteases from haloalkaliphilic microorganisms are biocatalyst with a great interest to be studied in biotechnological implications. Nevertheless, available methods for assay haloalkaliphilic proteases on protein substrates are few, are carry out to end point and implies the use of complicated steps. A new high throughput screening assay is described involving pH indicators at haloalkaline conditions. Rapid Enzymatic Determination method use casein as natural substrate and salts: NaCl and KCl to measure the activity of halophilic proteases. When a peptide bond is cleaved by a protease a proton is released changing the color of the indicator, proteolysis is followed by monitoring the decrease in absorbance. This is the easiest, quickest, and cheapest kinetic assay. Moreover, amounts of about 40 ng×mL-1 of proteolytic enzyme could be detected by this method in a pH range from 7.5 to 10.9 and salt concentrations from 0 to 4 M.
Keywords: Protease activity; Haloalkaline; Protease screening; Casein; pH-indicator.
|
|
 |

