 |
|
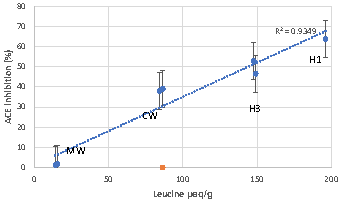
Bovine colostrum is an important source of nutritional and immunological factors that are vital for the early development and protection of the newborn. The aim of this study was to evaluate the antioxidant, mineral-binding (iron and calcium), and angiotensin-converting enzyme (ACE) inhibitory properties of the peptides generated through the enzymatic hydrolysis of bovine colostrum whey proteins. The whey proteins were hydrolyzed with pepsin at two pH values (1.3 and 2), and in a sequential hydrolysis system with pepsin and pancreatin. The hydrolysates were separated by ultrafiltration in three fractions with molecular weights of less than 10, from 10 to 30, and more than 30 kDa. The antioxidant and calcium binding activities increased with the hydrolysis and the highest values were obtained in the case of the fractions with less than 10 kDa. The fractions with molecular weights higher than 30 kDa had the best iron binding capacity. A high positive correlation (R=0.9669) was obtained between the degree of hydrolysis and the ACE inhibitory activity.
Keywords: bioactive peptides, bovine colostrum whey, antioxidant capacity, mineral binding capacity, ACE inhibition.
|
|
 |

