 |
|
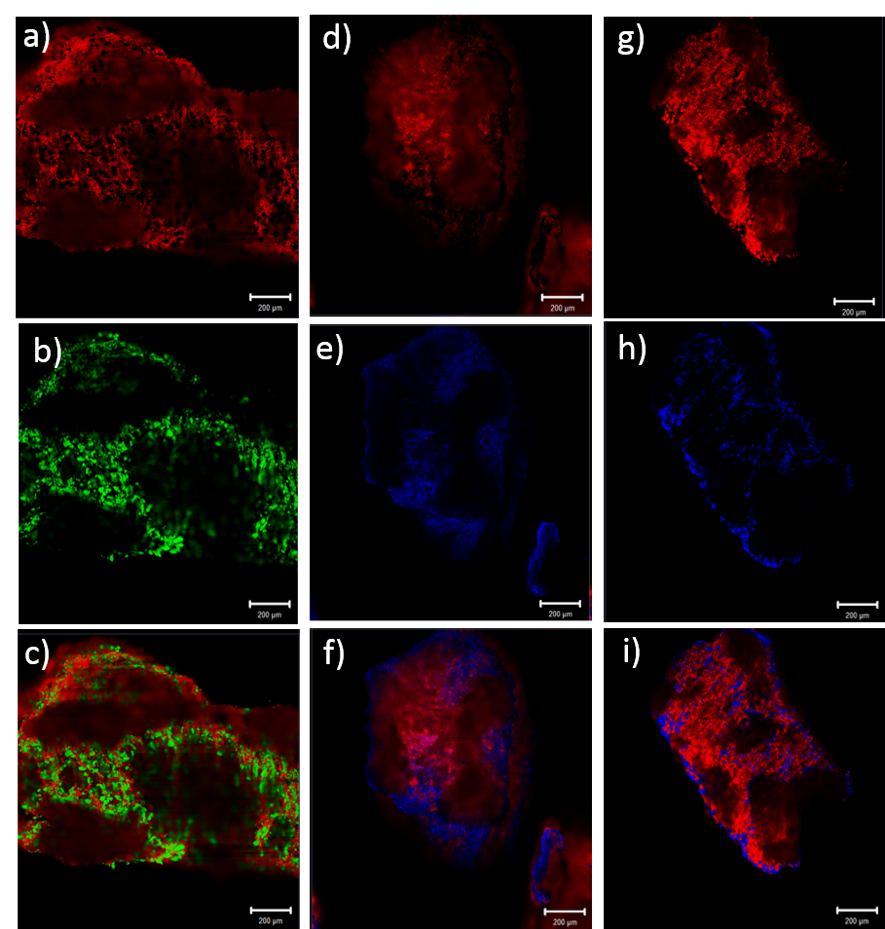
Lead (Pb2+) and copper (Cu2+) are polluting metals due to their toxicity; however, the extraction of these metals is essential for economic development, so it is important to look for efficient and low-cost alternatives that can remove heavy metals from the various bodies of water. One of the alternatives used in this work is biosorption, for which an agroindustrial waste (epidermis from Agave atrovirens) was used to evaluate the affinity of removal of lead and copper in aqueous solutions; in addition, spectroscopy and microscopy techniques were used to elucidate and corroborate the removal and affinity capacity of the agave epidermis for both metals studied. The optimal pH value for the removal of both metals was 3. The adsorption isotherms yielded a qmax of 25.7 and 8.6 mg/g for lead and copper, respectively. Adjusting to the Langmuir-Freundlich model, the adsorption kinetics were pseudo-second order, and it was found that the equilibrium time was at 140 min. The spectroscopy and microscopy analyses corroborated the affinity between metals and functional groups of the agave, as well as with the elemental analysis, which reported 17.38% of lead and 4.25% of copper.
Keywords: metals, biosorption, microscopy, spectroscopy, Agave epidermis waste.
|
|
 |

