 |
|
The thiourea leaching process for precious metals recovery has been studied as an alternative process to cyanidation. The accurate and simple determination of thiourea is important to guarantee the efficiency of the process and to evaluate the thiourea consumption during the gold and silver leaching from minerals; however, there is not, until now, any trustable method to determine the thiourea concentration for the high levels used in the extractive metallurgy industry. The present work, performed at CINVESTAV Saltillo, studies thermodynamically and experimentally the thiourea determination by iodate titration in the search of a precise technique appropriate for the high thiourea concentrations used in the leaching of precious metals.
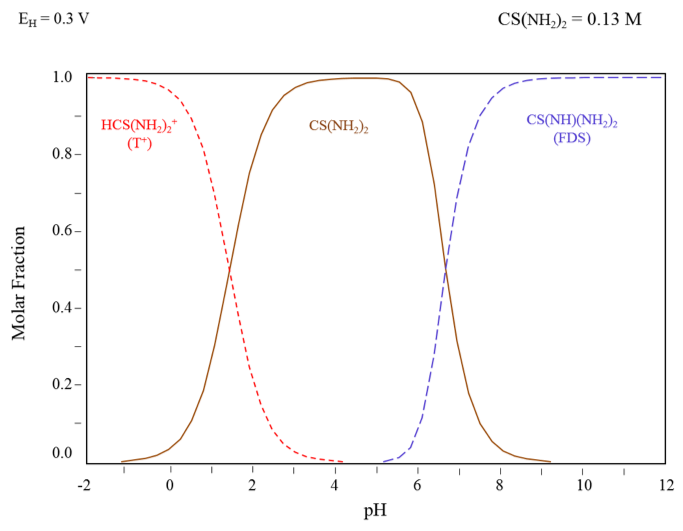
The thiourea titration by iodate in acid media permits the precise determination of high thiourea concentrations (up to 10,000 ppm). The acidification of thiourea (CS(NH2)2) produces protonated thiourea (HCS(NH2)2+) which reacts with iodate to form the protonated formamidine disulfide ([CS(NH2)2]22+). The presence of oxidant ions like cupric or ferric ions can oxidize the thiourea into formamidine disulfide (FDS2+), decreasing the leaching strength of the solution. When zinc dust is added to the solution before the titration, it reduces FDS2+ by regenerating the thiourea.
Keywords: thiourea determination, precious metals leaching, iodate titration, formamidine disulfide.
|
|
 |