 |
|
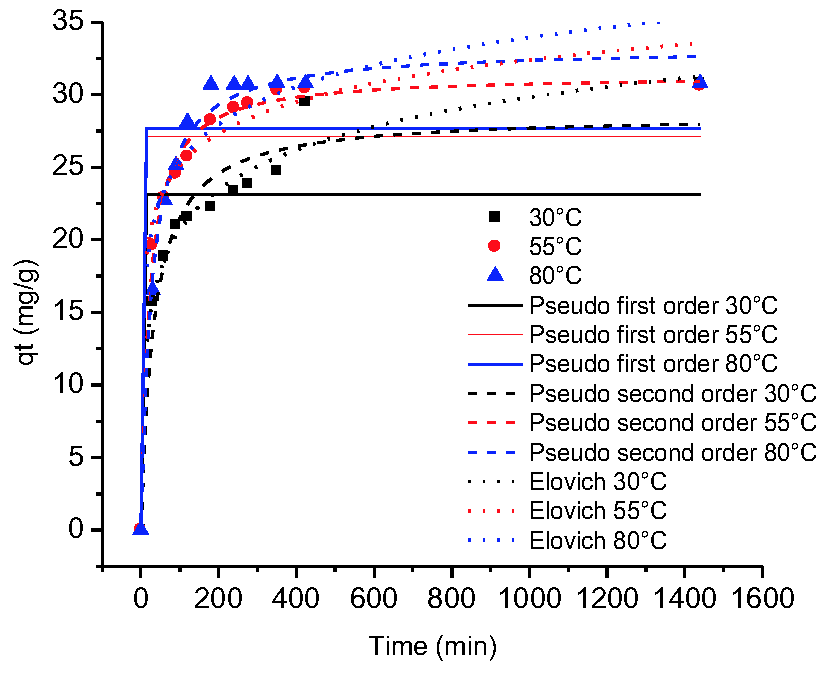
The presence of heavy metals in bodies of water is an environmental problem, due to its toxicity and bioaccumulation in ecosystems. The adsorption kinetics of Cr (VI) was studied in a batch system using plantain peels and oil palm bagasse, evaluating the effect of temperature and amount of adsorbent on the process. The bioadsorbents were placed in contact with the solution of Cr (VI) at pH 2 and 100 ppm, taking samples at different times until equilibrium. The kinetics were adjusted to the pseudo-first order, pseudo-second order and Elovich models. It was established that: the increase in temperature favours the process for the plantain peel and decreases the capacity of adsorption for oil palm bagasse; and that a decrease in the amount of adsorbent favours the kinetics for both biomasses studied. The Elovich model describes the behaviour for the plantain peel, while the pseudo-first and pseudo-second-order models do it for the oil palm bagasse when the temperature varies. The Elovich model better approximates the kinetic data by varying the adsorbent dose of both biomasses, suggesting that the process of adsorption of Cr (VI) was controlled by chemisorption.
Keywords: biomass, chromium, plantain peel, oil cane bagasse, remotion.
|
|
 |