 |
|
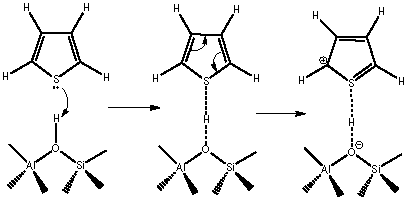
Due to the inconsistencies in the literature about the structure of thiophene oligomers, formed by the interaction between thiophene and the Brönsted acid sites of zeolite Y, the direct analysis of these oligomeric species is fundamental, mainly to understand how they are made up and their correct structure. The materials used were zeolite Y in its protonic (HY) and sodic (NaY) form. The interaction between thiophene and the zeolitic material was performed by the adsorption of thiophene at room temperature using a solution of thiophene in n-octane (100-700 ppmw), leading to the formation of cationic thiophene oligomers that are trapped inside the cavities of HY, among other non-oligomeric species. The thiophene-zeolite Y interaction occurs through the Brönsted acid sites by two possible routes: i) by electrophilic attack to the pair of free electrons in sulfur, forming oligothiophene whose size is limited by the structure of zeolite and, ii) by interaction with the α-carbon that leads to the formation of thiols and hydrocarbons with carbons with sp3and H2S hybridization.
Keywords: Thiophene, Zeolite Y, Brönsted acid sites, Oligomers.
|
|
 |

