 |
|
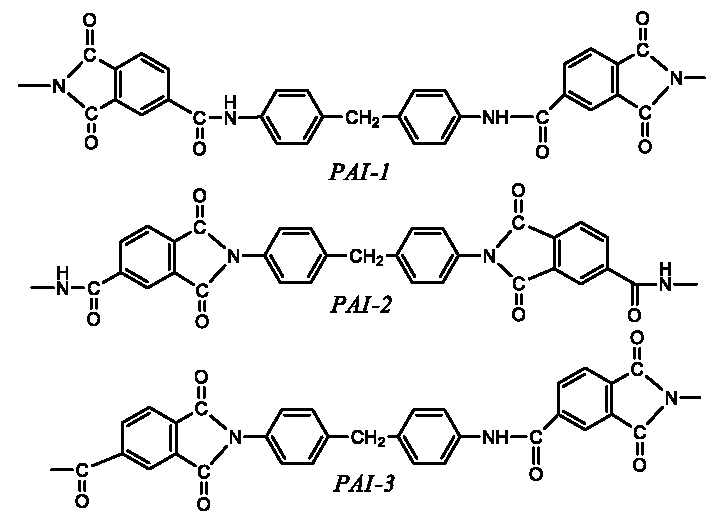
The influence in addition order of monomers for the polymerization reaction of polyamide imide (PAI), at a 1:1 molar ratio of TMA:MDI in NMP as a solvent was studied thoroughly. In the first instance, the chemical structure of the PAI was obtained using FTIR, 1H-NMR and Elemental analysis. On the other hand, the reaction kinetic followed by viscosimetry (measuring the instantaneous intrinsic viscosities at different times), was carried out on three synthesis routes proposed at 140 ℃ for 24 h of reaction: a) MDI / NMP solution to later add TMA, b) MDI / TMA solution in NMP and c) TMA / NMP solution to later add MDI, in order to determine some parameters such as reaction order and rate constant. The kinetic data analysis methods applied were the integral, the differential and Powell's, which showed that the reaction order was 2 and the velocity constant of k = 0.0006 M-1·s-1.
Keywords: order of addition, polyamide imide, instantaneous intrinsic viscosities, reaction kinetics, kinetic data analysis methods.
|
|
 |

