 |
|
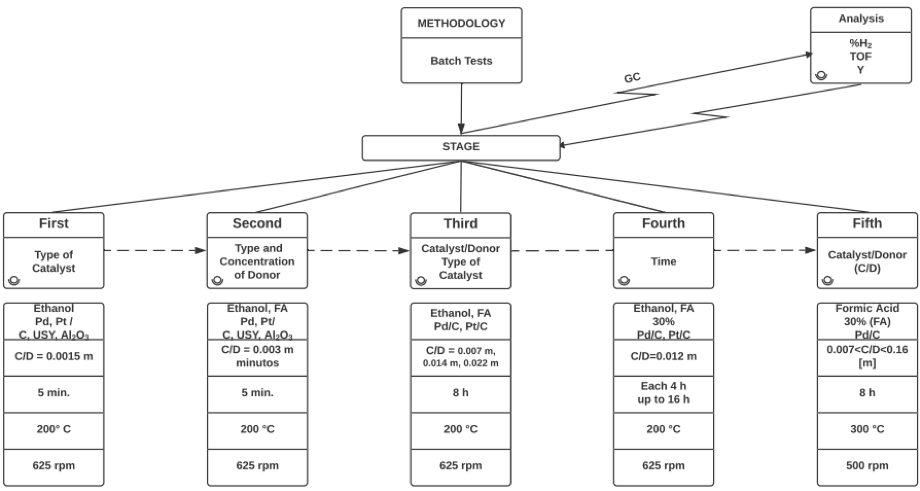
Many applications, including energy applications, require handling pressurized hydrogen gas. However, an alternative option allows the use of a donor that generates hydrogen in situ (either by transfer and / or catalytic reforming), instead of using molecular hydrogen. Ethanol and formic acid, which are biorefinery streams, were used as donors. The effect of metallic catalysts of Pd and Pt, supported on C, g-Al2O3 and zeolite USY, on the generation of hydrogen was evaluated using different ratios between the active phase and the donor, and different times and temperatures of reaction in a high pressure reactor (up to 1000 psi). The catalysts were characterized by DRX, SEM, FTIR and FRX. At the same time, High-Pressure Differential Scanning Calorimetry (HP DSC) was performed for the most promising systems with a ramp of 10 °C/min. Gas chromatography was used to analyze the production of hydrogen. Findings show that the system that generates more hydrogen, with 88%, is 5% Pd/C over 30% formic acid at 300 °C, 500 rpm and a ratio catalyst mass to donor from 1 to 10 (0.028 m).
Keywords: hydrogen, biodonor, catalytic transfer, ethanol, formic acid.
|
|
 |

