 |
|
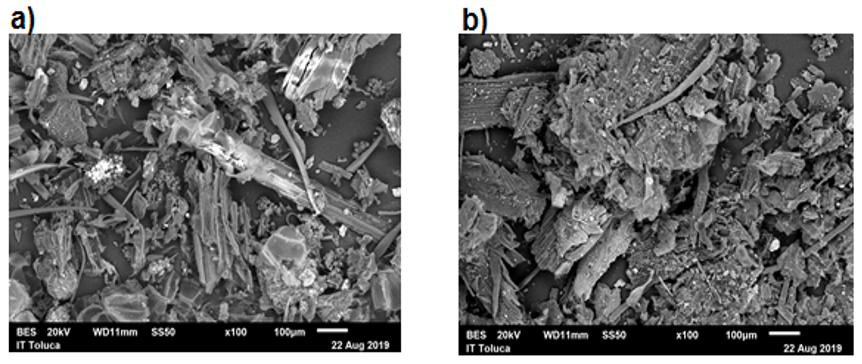
Dilute acid hydrolysis at high temperature is one technique to pretreat and produce monomeric sugars from lignocelluloses; however, its main disadvantage is the production of inhibitory aldehydes. In this paper, sugar production was realized by acid hydrolysis of corn stover employing 2.2 M hydrochloric acid solution and low temperature range (60 – 80 ºC). Experimental data were great fitted to different kinetic models, thermodynamic parameters were calculated from first order, Saeman and Saeman biphasic kinetic model, rate constants showed similar values between each other demonstrating that corn stover hydrolysis is an endothermic and non-spontaneous process capable to generate thermodynamically stable products. FTIR and SEM analyses showed the breaking of lignocellulosic matrix and the depolymerization of polysaccharides after acid treatment. The proposed operational conditions were adequate to produce reducing sugars avoiding decomposition into inhibitory aldehydes.
Keywords: Acid hydrolysis, corn stover, holocellulose, kinetic study, reducing sugars.
|
|
 |

