 |
|
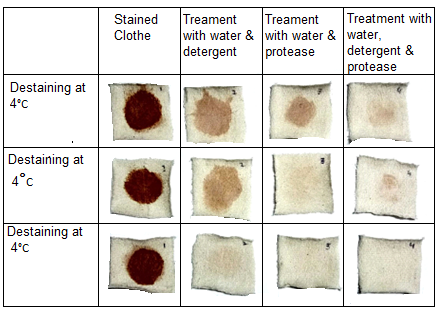
Cloning of protease gene from a thermophilic strain of Geobacillus stearothermophilus (B-1172) was carried out in E. coli BL 21, and its expression was studied. The expressed protease was purified followed by its identification. A 16.9 folds purification with 55.68% recovery of the protease was achieved by ammonium sulfate precipitation and gel filtration chromatography. The protease specific activity was 120 U mg−1. The purified enzyme remained stable at 90°C at a pH range 6-9. Its interaction with EDTA, different metal ions, inhibitors, surfactants and detergents was also mapped. Its interaction with EDTA showed no significant effect on the activity of the enzyme confirming its metaloprotease nature. Metal ions i.e. Ca2+, Mg2+, Ni2+, Cd2+, Cu2+, Zn2+ showed no significant effect on the stability of protease. Its compatibility was checked with different commercial detergent (6 mg/mL) such as Surf Excel Arial, Bonus, wheel and Shine. It retained more than 80% proteolytic activity in all detergents after incubation at 50°C for 1 h. Wash performance analysis of the protease of G. stearothermophilus showed good results of de-staining of blood sample at various temperatures. Therefore, recombinant protease could prove as good candidate for commercial use in detergents.
Keywords: Geobacillus stearothermophilus, protease, thermophile, detergent, purification.
|
|
 |