 |
|
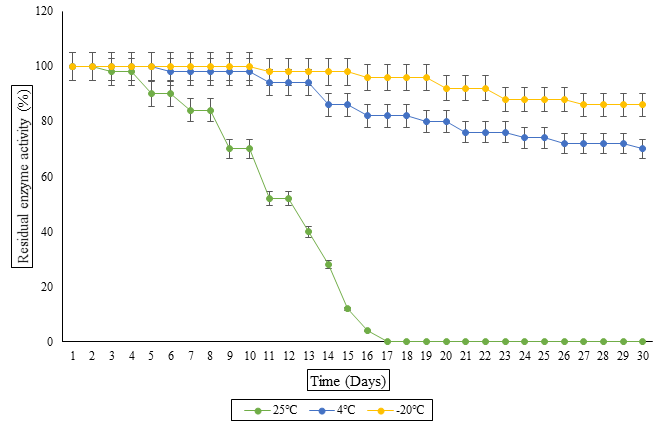
Increasing industrial demand has urged the scientists to biosynthesize polygalacturonase (PG) that would be operative, stable, cost effective and can be used in industrial applications particularly in fruit juice clarification and poultry feed. Polygalacturonase purification and characterization from A. tamarii was the main focus of the current research work. Fractionation by ammonium sulphate along with ion exchange chromatography was used for purification and 2.27 purification fold with 51.25 percentage yield was attained. SDS-PAGE analysis showed the molecular mass of purified enzyme as 70kDa. Enzyme kinetic assessment i.e. Km 2.85mg/mL and Vmax 55.55 in addition with thermodynamic determinants such as Ea=-39.84 KJ/mol, ΔH=37.62 KJ/mol and ΔS=-38.05 KJ/mol proves industrial stability of this enzyme. Further, different parameters were characterized which revealed that enzyme remained stable up to 50℃ and pH 8. Furthermore, PG found to retain its activity in the presence of different metals whereas some inhibitors reduced its activity i.e. PMSF and EDTA. The enzyme was found to have a better shelf life of 30 days at -20 and 4oC compared to room temperature. The reduction in turbidity of fresh juice and increase in body weight of chicks feeding on feed treated with purified PG proves its efficiency in industrial applications.
Keywords: Catalysis, Clarification, Stability, Thermodynamics, Kinetics.
|
|
 |

