 |
|
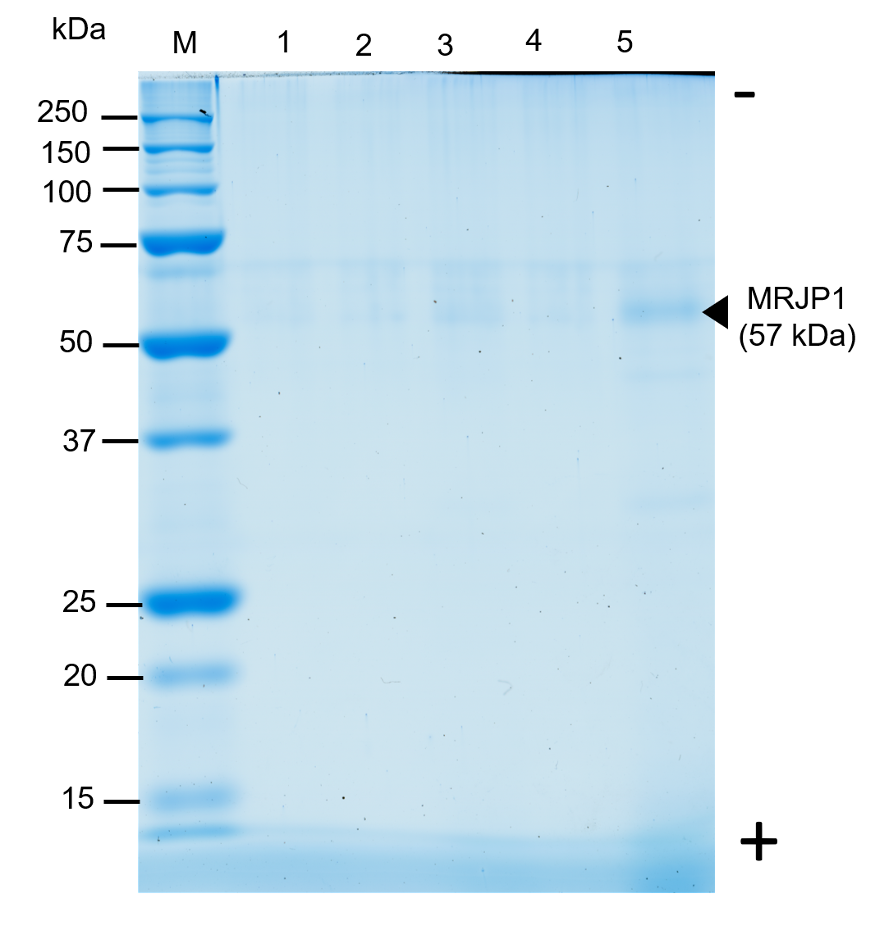
Major Royal Jelly Protein 1 (MRJP1) is the main protein component of the bee-produced complex mixture royal jelly, which is the only nutrient source for queen bees promoting increased lifespan, body size and fertility. Recombinant production of MRJP1 represents an alternative to direct extraction from royal jelly. Production in Pichia pastoris results in high density biomass, with a supernatant containing high amount of impurities. Various methods have been applied to recover and/or purify MRJP1. Here, exploiting the physicochemical properties of MRJP1, reverse phase chromatography (RPC), size exclusion chromatography (SEC) and ion-exchange chromatography (IEX) were investigated as alternative methods to recover MRJP1 directly from supernatant. All techniques showed a 57-kDa band in SDS-PAGE analysis, corresponding to the size of recombinant MRJP1, with contaminants attributed to culture media. However, SEC coupled to IEX evidenced a single peak in the chromatogram corresponding to MRJP1 which suggest it may be a good protocol to recover recombinant MRJP1 from P. pastoris supernatant. This approach serves as a procedure to identify MRJP1 in fermentation culture of P. pastoris. This is the first report about characterization of IEX-based recovery of recombinant Apis mellifera MRJP1 produced in Pichia pastoris without the use of histidine tags.
Keywords: MRJP1, Pichia pastoris, ion exchange chromatography, size exclusion chromatography.
|
|
 |

