 |
|
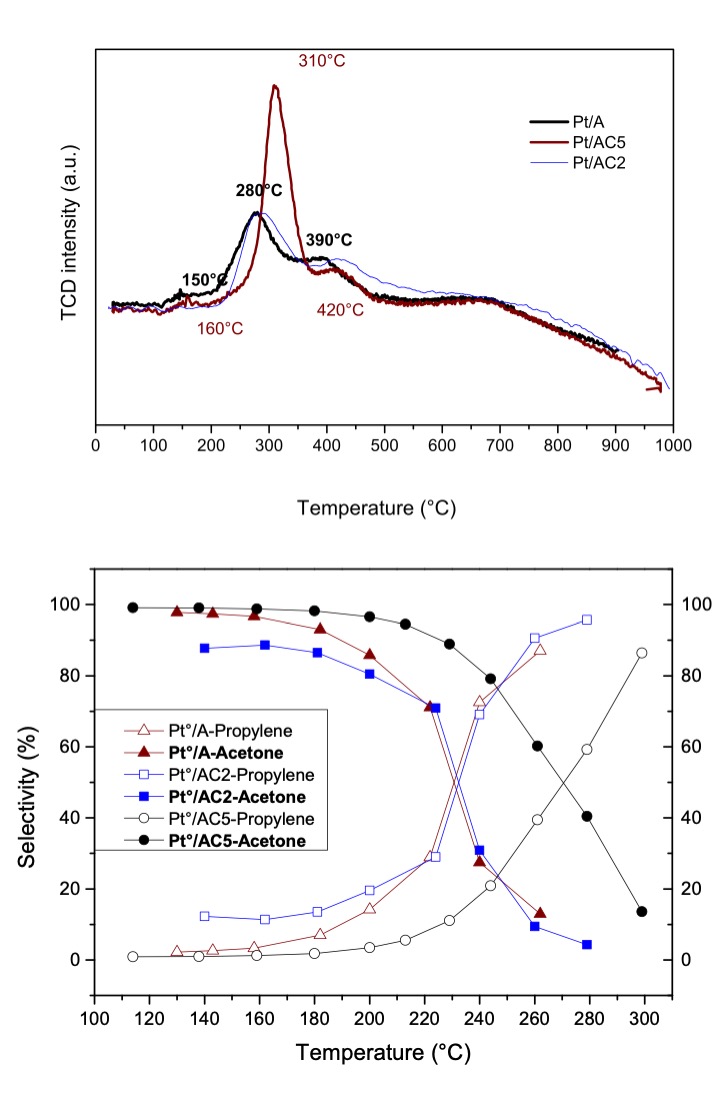
A series of Pt-based catalysts were tested in 2-propanol parallel reactions to produce mainly propylene and water plus acetone and hydrogen. Alumina and alumina-ceria catalytic supports (AC) were prepared by the sol-gel method. Pt catalysts were obtained by IWI and characterized by AAS, TPR, and FTIR. It was found the calcined catalysts (PtOx/AC) increased the dehydration capacity when compared to their respective catalytic supports, both series with no appreciable dehydrogenation products. The reduced catalysts (Pt0/AC) showed high dehydrogenation activity in 2-propanol reactions, with high selectivity towards acetone and hydrogen at low temperatures. These catalytic systems present a change in selectivity towards propylene above 230°C and the loss of activity of Pt-reduced species. This deactivation was related with the bands of asymmetric vibrations of C-H and C-O bonds in the post-reaction material characterized by FT-IR, which have been attributed to either carbon deposition or oxygenated intermediates chemisorbed on Pt surface. Moreover, the temperature range, where Pt0 is deactivated, is progressively shifted to higher values as the amount of ceria increases. This is attributed to the fact that ceria modifies the surface interactions of Pt sites with the molecules that cause deactivation. Catalysts were completely regenerated with a mild treatment in a N2 flow at 500ºC.
Keywords: Pt/Al2O3-CeO2, 2-propanol dehydrogenation, 2-propanol dehydration, Pt deactivation, Pt regeneration.
|
|
 |

