 |
|
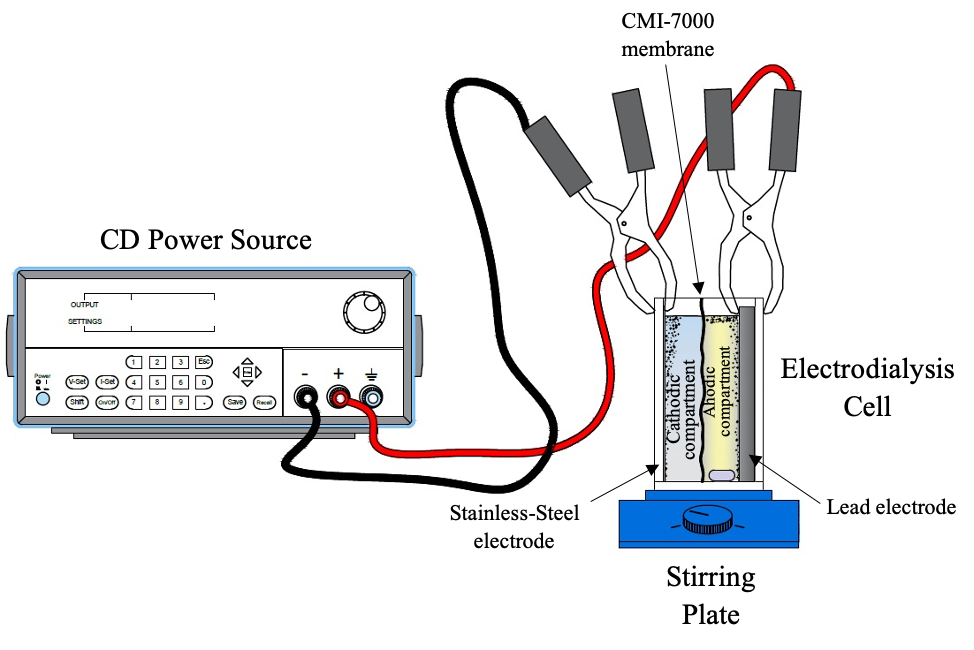
Iron is one of the main elements present in surface and ground waters due to mineral leaching or mining industry activities. To avoid the formation of acid mine drainage (AMD), it is necessary to treat the water with the presence of iron to reduce the environmental impact and obtain water that can be reused. In this work, electrodialysis is proposed for removing iron from a solution. To do this, it is necessary to know the appropriate conditions of current density, pH, and concentration to carry out the removal of iron in an analogous solution in concentration to those found in mineral processing. The electrodialysis tests were performed in a two-compartment cell. From the data obtained in the chemical analysis, the removal percentages, the amounts of mass removed, and the removal rates were calculated to determine the best operating parameters. Discussion is also made from the thermodynamics that determines the speciation of iron in the anolyte. The maximum percentage of iron removal reached was 97.15%, with the next parameters: current density of 390 A/m2, initial iron concentration (CFe) of 40 mg/L, initial pH value (pHi) of 4, agitation speed (v) of 500 r.p.m. and temperature (T) of 25°C.
Keywords: Iron, Electrodialysis, Removal, Current density, Diffusion.
|
|
 |

