 |
|
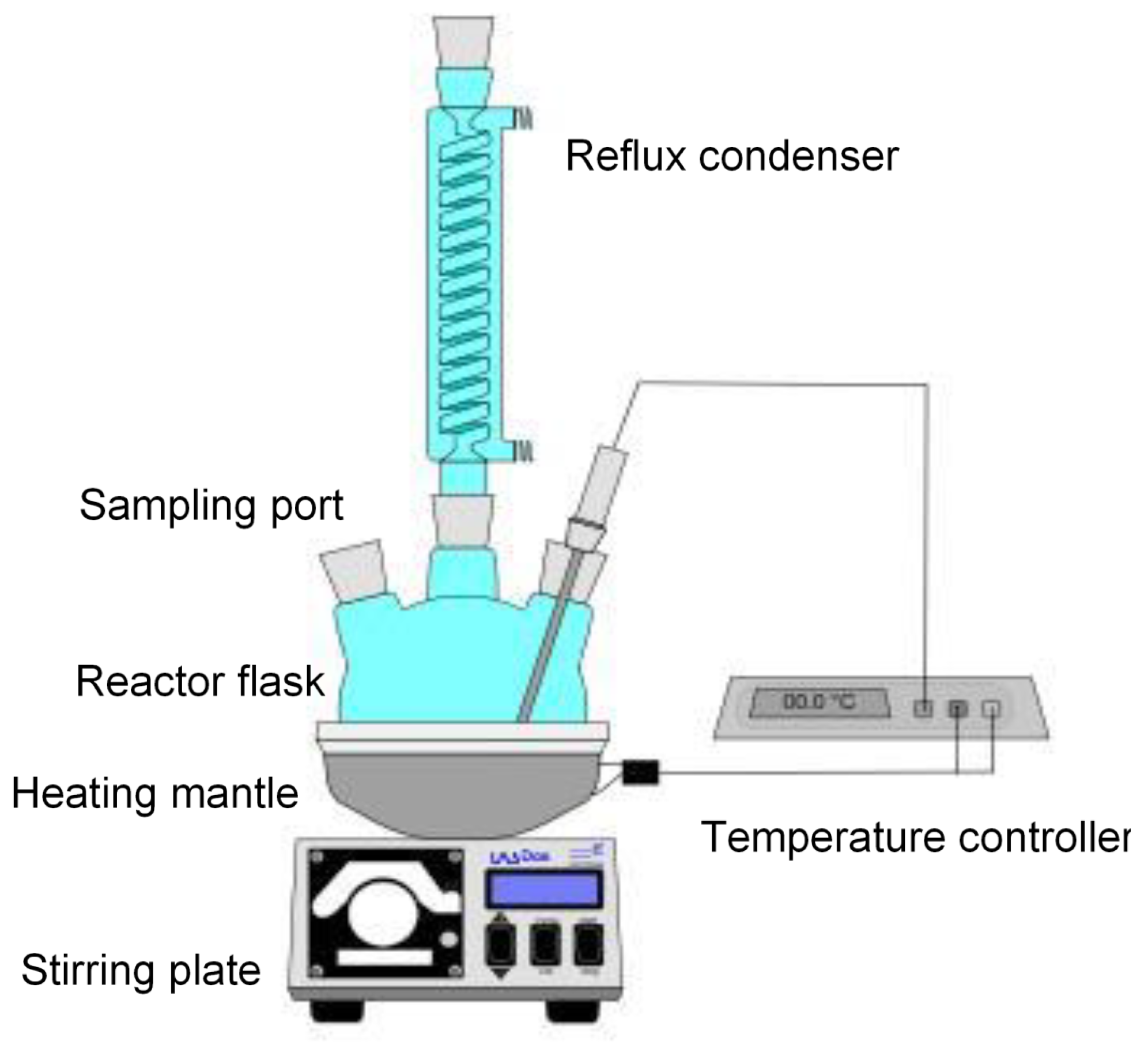
Glycol ether acetates are solvents of great industrial interest due to their low water solubility and slow evaporation rate. In the present work, the kinetics of esterification of glycol ethers with acetic acid to obtain glycol ether acetates was studied. Different synthesis conditions were evaluated, such as reaction temperature, catalyst type, catalyst loading and molar ratio between reactants. Uncatalyzed, catalyzed under homogeneous conditions with pTSA and under heterogeneous conditions with Amberlyst 15 were carried out. Second order kinetic model for the homogeneous system and Langmuir-Hinshelwood kinetic model for the heterogeneous system were proposed and the fit to the experimental data was performed to estimate the reaction rate constants. It was found that the maximum in reaction conversion depends mostly on the ratio between reactants, while temperature, catalyst type and catalyst loading have a significant effect on the reaction rate. Models’ predictions are in good agreement with the experimental data and the values of the reaction rate constants are consistent with values reported in other studies.
Keywords: glycol ether acetate, esterification, kinetics, Amberlyst 15, pTSA.
|
|
 |

