 |
|
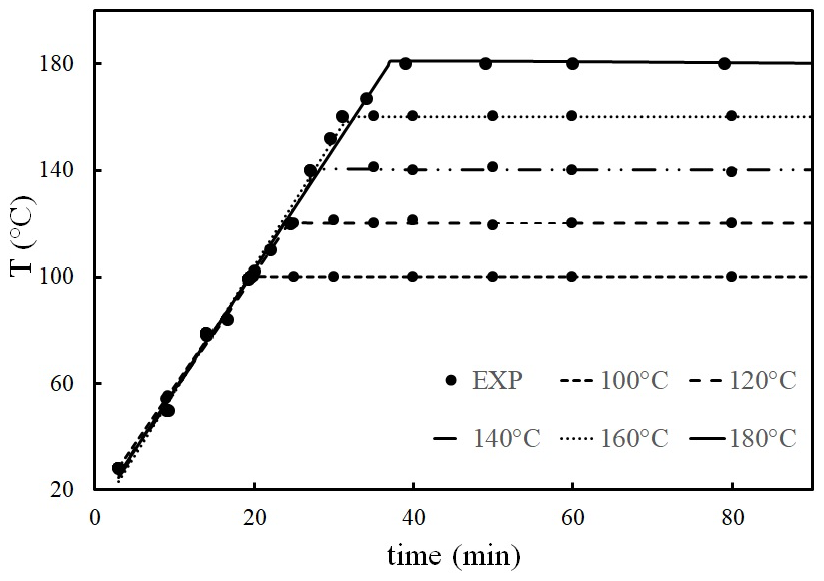
In this paper the composition change of Ipomoea arborescens was evaluated under acid hydrolysis pretreatment, and the kinetic parameters of this reaction were determined. The goal was to evaluate the effects of particle size in the reaction rate of hemicellulose hydrolysis, and to find the concentration of sulfuric acid in which the reaction mechanism favors hemicellulose hydrolysis of Ipomoea arborescens particles. Once the best conditions were found, Ipomoea arborescens particles were hydrolyzed at different temperatures. A pseudo-homogenous model was adopted to describe hemicellulose hydrolysis. The empirical formula of hemicellulose was determined experimentally to have 21 molecules of xylose, 5 molecules of glucose and 1 molecule of galactose. The mathematical model of hemicellulose hydrolysis is successful to describe the experimental results when the temperature is between 120 and 160°C.
Keywords: Hemicellulose, xylose, acid hydrolysis, rate constants, activation energy.
|
|
 |

