 |
|
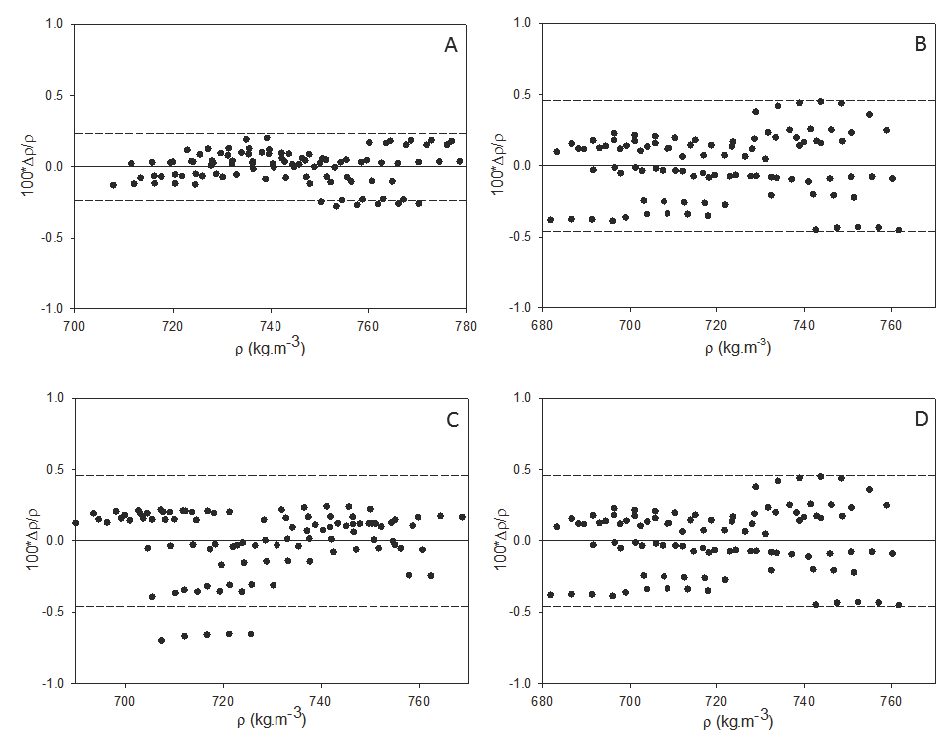
The objective of this work was to determine specific mass of ternary mixtures, using diotyl sodium sulfosuccinate (AOT) as a surfactant, iso-octane and hexane as organic solvent, and butanol and isopropanol as alcohol. The excess molar volume (VmE) was obtained only for binary systems of solvent and alcohol. It was observed that specific masses of pure liquids varied with temperature from 696.281 kg.m-3 (15oC) to 679.761 849 kg.m-3 (35oC) for iso-octane; 671.849 kg.m-3 (15°C) to 653.533 kg.m-3 (35°C), for hexane; 814.228 kg.m-3 (15°C) to 798.842 kg.m-3 (35°C), for butanol; 790.463 kg.m-3 (15°C) to 773.426 kg.m-3 (35°C) for isopropanol. The same was observed for binary mixtures, which presented the following variations: from 710.404 kg.m-3 (15°C) to 693.654 kg.m-3 (35°C), for iso-octane/butanol; from 706.286 kg.m-3 (15°C) to 688.972 kg.m-3 (35 °C), for iso-octane/isopropanol; from 700.970 kg.m-3 (15°C) to 682.817 kg.m-3 (35 °C), for hexane/butanol; from 694.169 kg.m-3 (15°C) to 675.125 kg.m-3 (35°C) for hexane/isopropanol. For VmE of binary mixtures, it was verified they varied from 15 to 35 °C, from -1.47504x10-6 m³.mol-1 to -1.50475x10-6 m³.mol-1 for iso-octane/butanol; from 2.09222x10-6 m³.mol-1 to 2.31939x10-6 m³.mol-1 for iso-octane/isopropanol; from -3.82449x10-6 m³.mol-1 to -4.04924x10-6 m³.mol-1 for hexane/butanol; from 4.34134x10-6 m³.mol-1 to 4.61050x10-6 m³.mol-1 for hexane/isopropanol.
Keywords: interactions, linear chain, branched chain, micelle.
|
|
 |

