 |
|
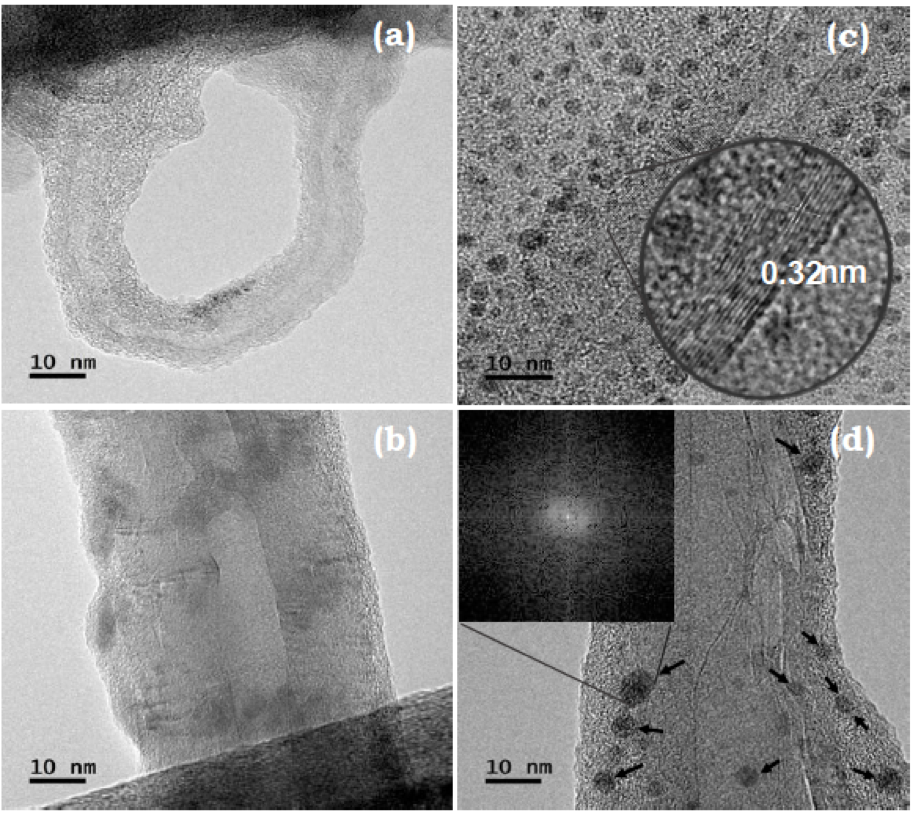
In the present work, it was studied the effect over the adsorption capacity of Cd(II) ions in carbon nanotubes (NTC) by adding several functional organic groups on its structure as: –OH, –C=O, –CH and –COOH, using as precursors: sulfuric acid, nitric acid, hydrogen peroxide, methanol, acetic acid and acetone. The modificated nanotubes (NTC'f) were characterized by transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffraction (XRD) and Fourier transform infrared (FTIR) to reveal the modified morphology and structure. In order to evaluate the adsorption performance of NTC'f for Cd(II) ions, a UV-Vis spectrophotometer was used. Several parameters were evaluated: Cd(II) ions concentration, solution pH and samples exposure time to NTC'f. Finally, a relationship between structure and functionality of the NTC'f and the Cd(II) ions adsoption was found, where the funtionalized nanotubes with -COOH and -OH exhibited a higher adsorption rate at room temperature, pH= 6, exposure time of 72 hours, where the NTC’f were able to absorb a 100 % of Cd(II) ions in a 50 mg.g-1 ratio.
Keywords: adsorption, functionalization, carbon nanotubes, cadmium ions, metal.
|
|
 |

